Human Cord Blood CD8+ Cytotoxic T Cells
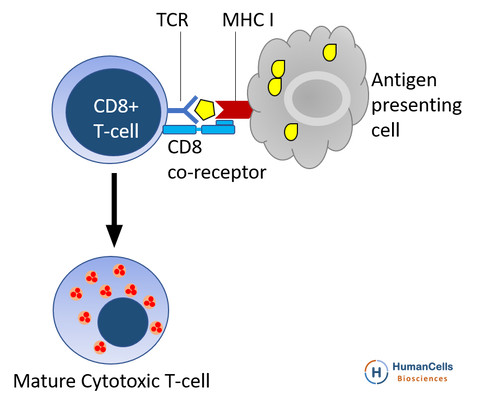
The CD8+ T cells, also known as Cytotoxic T cells (TC cells), kill any cells that present foreign antigen fragments, such as cancer cells, cells that are infected (particularly with viruses), or cells that are damaged in other ways. Most cytotoxic T cells express T-cell receptors (TCRs) that can recognize a specific antigen, and a glycoprotein called CD8 which binds to the constant portion of the class I MHC molecule. When exposed to infected/dysfunctional somatic cells, CD8+ T cells release the cytotoxins perforin, granzymes, and granulysin, which triggers the caspase cascade and eventually leads to apoptosis. CD8+ T cells have been implicated in the pathogenesis of hepatitis B virus infection, arthritis etc.
Our Human Cord Blood CD8+ cytotoxic T cells are isolated from cord blood mononuclear cells by immunomagnetic selection (positive selection). All cord blood is collected in Citric Phosphate with Dextrose Buffer (CPD) from fully consented IRB approved donors.

Most cytotoxic T cells express T-cell receptors (TCRs) that can recognize a specific antigen. An antigen is a molecule capable of stimulating an immune response, and is often produced by cancer cells or viruses. Antigens inside a cell are bound to class I MHC molecules, and brought to the surface of the cell by the class I MHC molecule, where they can be recognized by the T cell. If the TCR is specific for that antigen, it binds to the complex of the class I MHC molecule and the antigen, and the T cell destroys the cell.
In order for the TCR to bind to the class I MHC molecule, the former must be accompanied by a glycoprotein called CD8, which binds to the constant portion of the class I MHC molecule. Therefore, these T cells are called CD8+ T cells.
The affinity between CD8 and the MHC molecule keeps the TC cell and the target cell bound closely together during antigen-specific activation. CD8+ T cells are recognized as TC cells once they become activated and are generally classified as having a pre-defined cytotoxic role within the immune system. However, CD8+ T cells also have the ability to make some cytokines.
To function, CD8 forms a dimer, consisting of a pair of CD8 chains. The most common form of CD8 is composed of a CD8-α and CD8-β chain, both members of the immunoglobulin superfamily with an immunoglobulin variable (IgV)-like extracellular domain connected to the membrane by a thin stalk, and an intracellular tail. Less-common homodimers of the CD8-α chain are also expressed on some cells. The molecular weight of each CD8 chain is about 34 kDa. The structure of the CD8 molecule was determined by Leahy, D.J., Axel, R., and Hendrickson, W.A. by X-ray Diffraction at a 2.6A resolution.[1] The structure was determined to have an immunoglobulin-like beta-sandwich folding and 114 amino acid residues. 2% of the protein is wound into α-helices and 46% into β-sheets, with the remaining 52% of the molecules remaining in the loop portions.
References
- Leahy DJ, Axel R, Hendrickson WA (March 1992). "Crystal structure of a soluble form of the human T cell coreceptor CD8 at 2.6 A resolution". Cell. 68 (6): 1145–62. PMID 1547508. doi:10.1016/0092-8674(92)90085-Q.






